Sparing the details: charting the ligandable proteome for stereoselective interactions
As humanity grapples with a myriad of complex health challenges, the quest for more effective therapeutics remains a paramount endeavor. Traditional drug discovery methods often struggle to pinpoint precise interactions between biologically relevant proteins and small molecules, leading to significant development woes and delays. However, groundbreaking advancements in the realm of protein profiling are paving the way for a transformative approach that could revolutionize drug discovery as we know it.
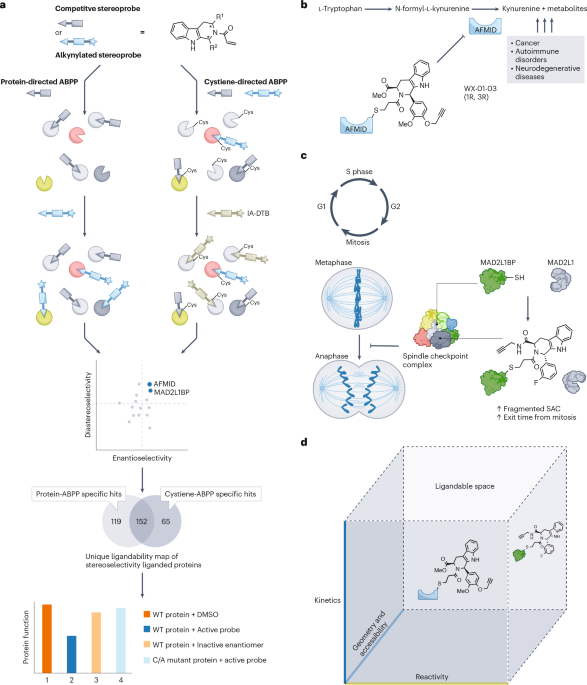
Recent research has unveiled innovative multi-tiered activity-based protein profiling techniques that forge comprehensive proteomic maps of chiral small-molecule interactions. By focusing on the ligandability of the human proteome, scientists are shedding light on the nuanced interactions that exist between various proteins and highly specific small molecules. In an astonishing breakthrough, over 300 distinct proteins have been identified that can bind to tryptoline acrylamides through both stereoselective and site-specific events, providing a roadmap for new therapeutic strategies.
Stereoselective interactions—where the spatial arrangement of atoms in a molecule directly influences its interaction with a target protein—are of crucial importance in biomedical science. These subtle variations can significantly affect a drug's efficacy and safety profile, underscoring why understanding such interactions is essential for targeted therapies.
A key innovation outlined in recent studies is the use of a paralog-hopping strategy, where researchers introduce electrophile-sensitive cysteines into cysteine-less paralogs. This creative tactic allows for screening with stereoprobe libraries, unveiling conserved pockets suitable for reversible small molecule binding—a noteworthy development as it broadens the field of potential drug targets beyond those that have been previously characterized.
Moreover, the implications of these findings extend beyond the laboratory. By elucidating reversible small molecule-binding pockets in the proteome, research like this significantly enhances our understanding of numerous biological processes, opening doors to the development of more selective and effective pharmaceutical agents. Such progress could lead to more successful treatments for a wide range of diseases, ultimately improving the quality of life for countless individuals.
In mental health, for instance, targeted therapies that leverage the complexities of stereoselective interactions might provide alternatives to the often hit-or-miss approaches currently used. Similarly, advancements in oncology could benefit substantially from the enriched understanding of ligandable proteins, offering novel strategies to combat resistant forms of cancer.
Ultimately, the shift from general drug design to a strategy that emphasizes precise protein-ligand interactions harbors the potential to create a wave of medicinal breakthroughs. By creating maps of the proteome and highlighting ligandability factors, future research and drug development bear the promise of catering to individual cellular biologies, making treatments not only more effective but also minimizing side effects.
In light of this promising frontier, connecting the dots between protein dynamics and therapeutic outcomes becomes an ever-more essential focus in biomedical research. Charting the ligandable proteome for stereoselective interactions isn’t just an exercise in molecular biology; it’s a direct call to action towards a more tailored and effective approach to health care—one that could very well lead us to a more enlightened era of therapeutic intervention and disease management for humanity.